Merkel Cell Carcinoma Market Set for Rapid Growth in the Coming 10 Years Across the 7MM | DelveInsight
The market size of Merkel cell carcinoma in the 7MM is expected to increase during the forecast period (2025–2034) due to several factors such as rising global incidence of MCC, rising awareness about mMCC among healthcare professionals and the general public is contributing to earlier diagnosis and treatment initiation, and substantial progress in the development of innovative treatment modalities for mMCC, including immunotherapies and targeted therapies.
New York, USA, Aug. 20, 2025 (GLOBE NEWSWIRE) -- Merkel Cell Carcinoma Market Set for Rapid Growth in the Coming 10 Years Across the 7MM | DelveInsight
The market size of Merkel cell carcinoma in the 7MM is expected to increase during the forecast period (2025–2034) due to several factors such as rising global incidence of MCC, rising awareness about mMCC among healthcare professionals and the general public is contributing to earlier diagnosis and treatment initiation, and substantial progress in the development of innovative treatment modalities for mMCC, including immunotherapies and targeted therapies.
DelveInsight’s Merkel Cell Carcinoma Market Insights report includes a comprehensive understanding of current treatment practices, emerging Merkel cell carcinoma drugs, market share of individual therapies, and current and forecasted Merkel cell carcinoma market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
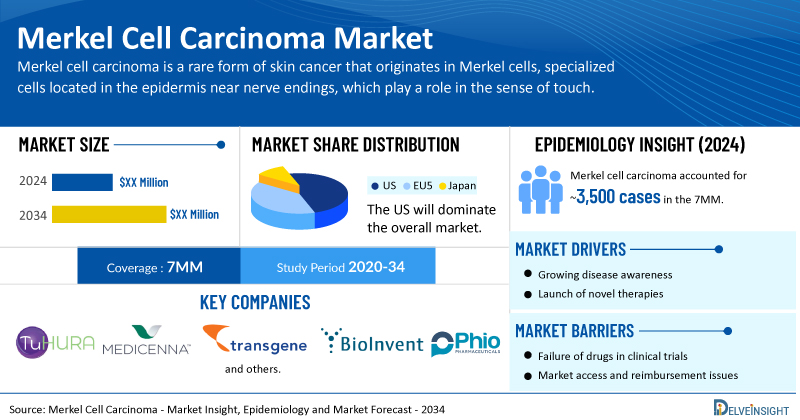
Key Takeaways from the Merkel Cell Carcinoma Market Report
- According to DelveInsight’s analysis, the total Merkel cell carcinoma treatment market size is expected to grow positively by 2034.
- The United States accounts for the largest market size of Merkel cell carcinoma, in comparison to EU4 (Germany, Italy, France, and Spain) and the UK, and Japan.
- According to Delveinsight, in 2024, Merkel cell carcinoma accounted for approximately 3,500 cases in the 7MM.
- Prominent companies, including TuHURA Biosciences, Medicenna Therapeutics, Transgene, BioInvent, Memgen, Phio Pharmaceuticals, and others, are actively working on innovative Merkel cell carcinoma drugs.
- Some of the key Merkel cell carcinoma therapies in the pipeline include IFx-Hu2/IFx-2.0, MDNA11, BT-001, MEM-288, PH-762, and others. These novel Merkel cell carcinoma therapies are anticipated to enter the Merkel cell carcinoma market in the forecast period and are expected to change the market.
Discover which Merkel cell carcinoma medications are expected to grab the market share @ Merkel Cell Carcinoma Market Report
Merkel Cell Carcinoma Market Dynamics
The Merkel cell carcinoma market dynamics are anticipated to change in the coming years. The MCC market is primarily driven by the rising incidence of the disease, fueled by an aging population, increased UV light exposure, and higher prevalence of immunosuppression-related conditions. Advances in diagnostic technologies, including improved imaging techniques and molecular testing for Merkel cell polyomavirus (MCPyV), are enabling earlier detection and targeted treatment approaches. Furthermore, the growing adoption of immunotherapies, particularly PD-(L)1 inhibitors, has significantly expanded treatment options, improving survival outcomes and boosting market growth. Increased awareness among healthcare providers, coupled with ongoing clinical trials exploring novel agents and combination therapies, is further accelerating the market’s expansion.
Furthermore, many potential therapies are being investigated for the treatment of Merkel cell carcinoma, and it is safe to predict that the treatment space will significantly impact the Merkel cell carcinoma market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the Merkel cell carcinoma market in the 7MM.
However, several factors may impede the growth of the Merkel cell carcinoma market. MCC is a rare and aggressive skin cancer, which leads to low disease awareness among both patients and healthcare providers, often resulting in delayed or missed diagnoses. The small patient population poses challenges for conducting large-scale clinical trials, slowing the development and regulatory approval of novel therapies. High treatment costs, particularly for advanced immunotherapies, restrict access in cost-sensitive markets and create reimbursement hurdles. Additionally, the limited availability of diagnostic expertise, especially in low- and middle-income countries, and the scarcity of targeted therapeutic options beyond immune checkpoint inhibitors further constrain market expansion.
Moreover, Merkel cell carcinoma treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the Merkel cell carcinoma market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the Merkel cell carcinoma market growth.
Merkel Cell Carcinoma Treatment Market
The leading therapeutic approach for metastatic Merkel cell carcinoma is immune checkpoint inhibitors (ICIs), which have shown notable clinical benefit. If the cancer reappears at its original skin site, surgical removal with wider margins is often attempted. This may be followed by radiation therapy if it has not been previously administered. In cases where surgery is not feasible, radiation therapy alone may be considered.
Research indicates that immunotherapy offers significant advantages for patients with advanced disease and outperforms other treatment options; however, many advanced MCC cases do not respond to PD-1/PD-L1 inhibitors. Currently approved MCC treatments include BAVENCIO (avelumab, Merck KGaA), KEYTRUDA (pembrolizumab, Merck), and ZYNYZ (retifanlimab-dlwr, Incyte).
ZYNYZ, an intravenous PD-1 inhibitor, is approved in the U.S. for adults with metastatic or recurrent locally advanced MCC. The U.S. FDA granted accelerated approval for this indication in March 2023, based on tumor response rates and duration of response. Incyte holds the global rights to ZYNYZ through an exclusive collaboration and licensing agreement with MacroGenics.
BAVENCIO works by disrupting cancer cell growth and spread. It is approved for adults and children aged 12 years and above with metastatic MCC, as well as for certain advanced bladder and urinary tract cancers that have progressed after platinum-based chemotherapy. In March 2017, the FDA granted accelerated approval for BAVENCIO in metastatic MCC patients aged 12 and older, based on results from the open-label, single-arm, multi-center JAVELIN Merkel 200 trial, which showed a clinically meaningful and durable overall response rate in patients with histologically confirmed metastatic MCC who had progressed after prior chemotherapy.
Learn more about the Merkel cell carcinoma treatment options @ Merkel Cell Carcinoma Treatment Guidelines
Merkel Cell Carcinoma Emerging Drugs and Companies
Pharmaceutical companies developing therapies for treating MCC include TuHURA Biosciences (IFx-Hu2/IFx-2.0), Transgene and BioInvent (BT-001), Memgen (MEM-288), Medicenna Therapeutics (MDNA11), Phio Pharmaceuticals (PH-762), and others with their candidates in different stages of clinical development.
IFx-2.0 is a personalized innate immune activator designed to stimulate the body’s natural defenses against cancer. The therapy involves injecting a small amount of plasmid DNA (pDNA) directly into a patient’s tumor. This pDNA encodes an immunogenic bacterial protein that becomes expressed on the tumor surface, prompting the immune system to recognize the tumor as foreign. By imitating a bacterial infection, IFx-2.0 turns the tumor itself into a trigger for immune activation, enabling an attack even on tumors that have previously evaded detection.
In June 2025, TuHURA Biosciences began a Phase III accelerated approval trial of IFx-2.0 in patients with advanced or metastatic Merkel cell carcinoma (MCC). This follows the company’s October 2024 announcement of its completed merger with Kintara Therapeutics, after which the combined entity retained the TuHURA Biosciences name. The company plans to launch a single Phase III accelerated approval registration study in the first half of 2025 for first-line MCC, under a Special Protocol Assessment (SPA) agreement with the U.S. FDA.
At the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting in May 2024, TuHURA presented Phase Ib trial results (NCT04160065) for IFx-Hu2.0, showing the therapy was safe and well-tolerated when administered once weekly for three weeks.
BT-001 is an oncolytic vaccinia virus engineered for selective replication in tumor cells and modified to produce GM-CSF along with a novel full-length anti-CTLA-4 human IgG1 monoclonal antibody. The therapy is currently in Phase I/II clinical development as part of a 50/50 co-development partnership between Transgene and BioInvent.
According to Transgene’s latest corporate presentation, Phase I data for BT-001 is expected in the second half of 2025. Preliminary results shared by Transgene and BioInvent at ESMO 2024 indicated that BT-001, both as a monotherapy and in combination with intravenous pembrolizumab, was well tolerated and demonstrated antitumor activity, including in tumors resistant to PD-(L)1 inhibitors.
The anticipated launch of these emerging Merkel cell carcinoma therapies are poised to transform the Merkel cell carcinoma market landscape in the coming years. As these cutting-edge Merkel cell carcinoma therapies continue to mature and gain regulatory approval, they are expected to reshape the Merkel cell carcinoma market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for Merkel cell carcinoma, visit @ Merkel Cell Carcinoma Management
Recent Developments in the Merkel Cell Carcinoma Market
- In June 2025, TuHURA Biosciences initiated its Phase III accelerated approval trial of IFx-2.0 in patients with advanced or metastatic MCC.
- In April 2025, a Safety Monitoring Committee (SMC) recommended dose-escalation for a Phase Ib trial (NCT06014086) evaluating PH-762 in cutaneous squamous cell carcinoma, melanoma, and MCC.
- In January 2025, Phio Pharmaceuticals announced promising results from the second cohort of its ongoing clinical study of PH-762, reporting pathologic responses that include two patients achieving a complete response with 100% tumor clearance.
Merkel Cell Carcinoma Overview
Merkel cell carcinoma is a rare form of skin cancer that originates in Merkel cells, specialized cells located in the epidermis near nerve endings, which play a role in the sense of touch. MCC development is linked either to the presence of clonally integrated Merkel cell polyomavirus (MCPyV, also known as human polyomavirus 5) or to prolonged exposure to ultraviolet (UV) light.
Managing and treating MCC typically requires a multidisciplinary approach, involving dermatologists, medical oncologists, surgical oncologists, radiation oncologists, oncology nurses, dietitians, psychiatrists, and other healthcare specialists. Because MCC is rare and carries a relatively high risk, comprehensive psychosocial support for patients and their families is often necessary. The primary diagnostic method is a skin biopsy. Additional tests, such as ultrasound, CT scan, PET-CT, or MRI, are performed to determine whether the cancer has spread to other areas of the body.
Merkel Cell Carcinoma Epidemiology Segmentation
The Merkel cell carcinoma epidemiology section provides insights into the historical and current Merkel cell carcinoma patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The Merkel cell carcinoma market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Incident Cases of MCC
- Gender-specific Incident Cases of MCC
- Age-specific Cases Incident of MCC
- Stage-specific Cases Incident of MCC
- Total Treatable Cases of MCC in the First-line and Second-line
Download the report to understand which factors are driving Merkel cell carcinoma epidemiology trends @ Merkel Cell Carcinoma Treatment Algorithm
| Merkel Cell Carcinoma Report Metrics | Details |
| Study Period | 2020–2034 |
| Merkel Cell Carcinoma Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Key Merkel Cell Carcinoma Companies | TuHURA Biosciences, Medicenna Therapeutics, Transgene, BioInvent, Memgen, Phio Pharmaceuticals, Incyte, Merck, and others |
| Key Merkel Cell Carcinoma Therapies | IFx-Hu2/IFx-2.0, MDNA11, BT-001, MEM-288, PH-762, ZYNYZ, KEYTRUDA, BAVENCIO, and others |
Scope of the Merkel Cell Carcinoma Market Report
- Merkel Cell Carcinoma Therapeutic Assessment: Merkel Cell Carcinoma current marketed and emerging therapies
- Merkel Cell Carcinoma Market Dynamics: Conjoint Analysis of Emerging Merkel Cell Carcinoma Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Merkel Cell Carcinoma Market Access and Reimbursement
Discover more about Merkel cell carcinoma drugs in development @ Merkel Cell Carcinoma Clinical Trials
Table of Contents
| 1 | Key Insights |
| 2 | Report Introduction |
| 3 | Executive Summary |
| 4 | Key Events |
| 5 | Epidemiology and Market Methodology |
| 6 | MCC: Market Overview at a Glance |
| 6.1 | Total Market Share (%) Distribution of MCC by Therapies in 2024 |
| 6.2 | Total Market Share (%) Distribution of MCC by Therapies in 2034 |
| 7 | Disease Background and Overview: MCC |
| 7.1 | Introduction |
| 7.2 | Risk Factors |
| 7.3 | Symptoms |
| 7.4 | Pathophysiology and disease pathways |
| 7.5 | Diagnostic Tests: Biomarker assays |
| 8 | Treatment and Guidelines |
| 8.1 | Current Treatment Landscape |
| 9 | Epidemiology and Patient Population |
| 9.1 | Key Findings |
| 9.2 | Assumptions and Rationale |
| 9.3 | Total Incident Cases of MCC in the 7MM |
| 9.4 | The United States |
| 9.4.1 | Total Incident Cases of MCC in the United States |
| 9.4.2 | Stage-specific Cases of MCC in the United States |
| 9.4.3 | Gender-specific Cases of MCC in the United States |
| 9.4.4 | Biomarker-specific Cases of MCC in the United States |
| 9.4.5 | Total Treatable Cases of MCC in the United States |
| 9.5 | EU4 and the UK |
| 9.6 | Japan |
| 10 | Patient Journey |
| 11 | Marketed Drugs |
| 11.1 | Key Competitors |
| 11.2 | ZYNYZ (retifanlimab-dlwr): Incyte |
| 11.2.1 | Product Description |
| 11.2.2 | Regulatory Milestones |
| 11.2.3 | Other Developmental Activities |
| 11.2.4 | Clinical Development Activity |
| 11.2.4.1 | Clinical Trial Information |
| 11.2.5 | Safety and Efficacy |
| 11.2.6 | Analyst Views |
| 11.3 | BAVENCIO (avelumab): Merck KGaA (EMD Serono) |
| List to be continued in the report… | |
| 12 | Emerging Therapies |
| 12.1 | Key Competitors |
| 12.2 | IFx-2.0: TuHURA Biosciences |
| 12.2.1 | Product Description |
| 12.2.2 | Other Developmental Activities |
| 12.2.3 | Clinical Development Activity |
| 12.2.3.1 | Clinical Trial Information |
| 12.2.4 | Safety and Efficacy |
| 12.2.5 | Analyst Views |
| 12.3 | BT-001: Transgene and BioInvent |
| List to be continued in the report… | |
| 13 | MCC: Market Size |
| 13.1 | Key Findings |
| 13.2 | Market Outlook |
| 13.3 | Conjoint Analysis |
| 13.4 | Key Market Forecast Assumptions |
| 13.4.1 | Cost Assumptions and Rebates |
| 13.4.2 | Pricing Trends |
| 13.4.3 | Analogue Assessment |
| 13.4.4 | Launch Year and Therapy Uptakes |
| 13.5 | Total Market Size of MCC in the 7MM |
| 13.6 | The United States Market Size |
| 13.6.1 | Total Market Size of MCC in the United States |
| 13.6.2 | Total Market Size of MCC by Therapies in the United States |
| 13.7 | EU4 and the UK Market Size |
| 13.8 | Japan Market Size |
| 14 | Unmet Needs |
| 15 | SWOT Analysis |
| 16 | KOL Views |
| 17 | Market Access and Reimbursement |
| 18 | Bibliography |
| 19 | Report Methodology |
Related Reports
Merkel Cell Carcinoma Epidemiology Forecast
Merkel Cell Carcinoma Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology as well as the Merkel cell carcinoma epidemiology trends.
Merkel Cell Carcinoma Pipeline
Merkel Cell Carcinoma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Merkel cell carcinoma companies, including ImmunityBio, OncoSec Medical, Exelixis, 4SC, Kartos therapeutics, Incyte corporation, Amgen, BioInvent International AB, SOTIO Biotech, Xencor, Exicure, Checkmate Pharmaceuticals, Takeda, Genocea Biosciences, NeoImmuneTech, Sensei Biotherapeutics, among others.
Metastatic Merkel Cell Carcinoma Market
Metastatic Merkel Cell Carcinoma Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key metastatic Merkel cell carcinoma companies, including Incyte Corporation, Merck, Pfizer, Millennium Pharmaceuticals Inc., Novartis, Exelixis, Bristol-Myers Squibb, EMD Serono, among others.
Metastatic Merkel Cell Carcinoma Pipeline
Metastatic Merkel Cell Carcinoma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key metastatic Merkel cell carcinoma companies, including Incyte Corporation, Transgene, Ocellaris Pharma, Roche, Exelixis, Sensei Biotherapeutics, Checkmate Pharmaceuticals, SOTIO Biotech, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
